

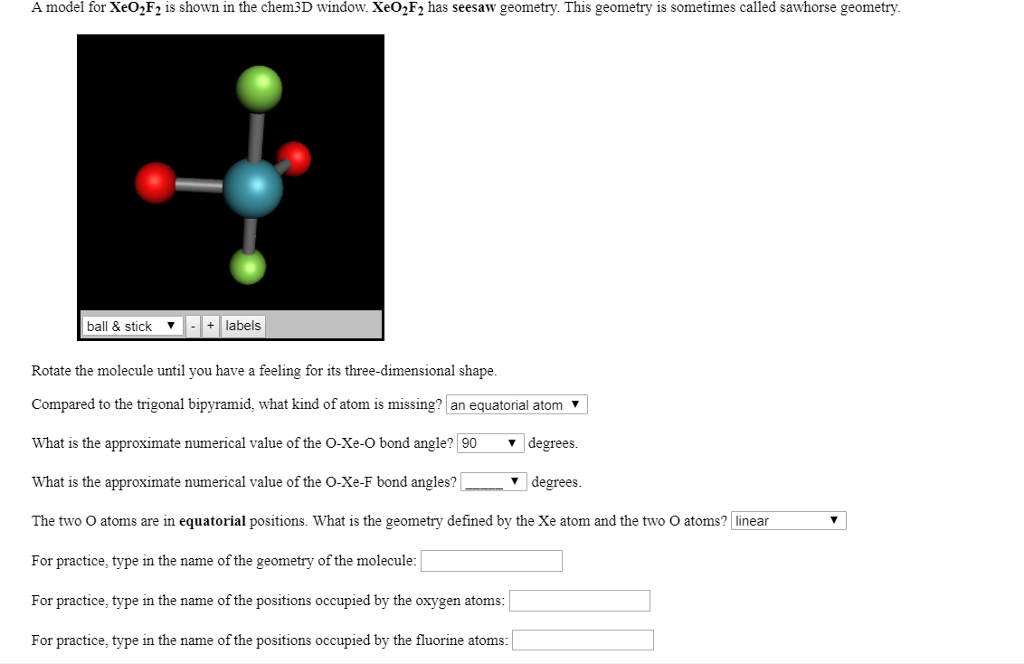
ISBN 981-238-153-8.For the seesaw shape, we have 5 regions of electron density (trigonal bipyramidal), consisting of 4 bonding pairs and 1 lone pair. A Bridge not Attacked: Chemical Warfare Civilian Research During World War II. Encyclopedia of Reagents for Organic Synthesis. "A simplified and efficient bromine-facilitated SF 4-preparation method".

National Institute for Occupational Safety and Health (NIOSH). ^ a b c d NIOSH Pocket Guide to Chemical Hazards.Toxicity Ĥ reacts inside the lungs with moisture, generating sulfur dioxide and hydrogen fluoride: SF 4 + 2 H 2O → SO 2 + 4 HF References This reaction proceeds via the intermediacy of thionyl fluoride, which usually does not interfere with the use of SF 4 as a reagent. Hydrolysis of SF 4 gives sulfur dioxide: SF 4 + 2 H 2O → SO 2 + 4 HF This reagent is prepared from SF 4: SF 4 + Me 3SiNEt 2 → Et 2NSF 3 + Me 3SiF Other reactions ĥCl), a useful source of the SF 5 group, is prepared from SF 4. The use of SF 4 is being superseded in recent years by the more conveniently handled diethylaminosulfur trifluoride, Et 2NSF 3, "DAST", where Et = CH 3CH 2. The coproducts from these fluorinations, including unreacted SF 4 together with SOF 2 and SO 2, are toxic but can be neutralized by their treatment with aqueous KOH. Hexafluoro-2-butyne can be similarly produced from acetylenedicarboxylic acid. For example, treatment of heptanoic acid with SF 4 at 100–130 ☌ produces 1,1,1-trifluoroheptane.

Carboxylic acids convert to trifluoromethyl derivatives. Also diols can give cyclic sulfite esters, (RO) 2SO. The presence of protons alpha to the carbonyl leads to side reactions and diminished (30–40%) yield. Ketones and aldehydes give geminal difluorides. Certain alcohols readily give the corresponding fluorocarbon. In organic synthesis, SF 4 is used to convert COH and C=O groups into CF and CF 2 groups, respectively. 20–86 ☌) method of producing SF 4 at high yield, without the requirement for reaction medium, has been demonstrated utilizing bromine (Br 2) instead of chlorine (Cl 2), S and KF: S + (2 + x) Br 2 + 4 KF → SF 4↑ + x Br 2 + 4 KBr Use of SF 4 for the synthesis of fluorocarbons Īlternatively, SF 4 at high yield is produced using sulfur (S), NaF and chlorine (Cl 2) in the absence of reaction medium, also at less-desirable elevated reaction temperatures (e.g. SF 4 is also produced in the absence of solvent at elevated temperatures. SF 4 is produced by the reaction of SCl 2 and NaF in acetonitrile: 3 SCl 2 + 4 NaF → SF 4 + S 2Cl 2 + 4 NaCl Intramolecular dynamic equilibration of SF 4. The 19F NMR spectrum of SF 4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation. Further contrasting with SF 4, SF 6 is extraordinarily inert chemically. In contrast to SF 4, the related molecule SF 6 has sulfur in the 6+ state, no valence electrons remain nonbonding on sulfur, hence the molecule adopts a highly symmetrical octahedral structure. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The relevant bond distances are S–F ax = 164.3 pm and S–F eq = 154.2 pm. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. The structure of SF 4 can therefore be anticipated using the principles of VSEPR theory: it is a see-saw shape, with S at the center. Of sulfur's total of six valence electrons, two form a lone pair. Sulfur in SF 4 is in the formal +4 oxidation state. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.
It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Sulfur tetrafluoride is the chemical compound with the formula S F 4.


 0 kommentar(er)
0 kommentar(er)
